Untitled Reusable Block
2024-07-27 12:31:36
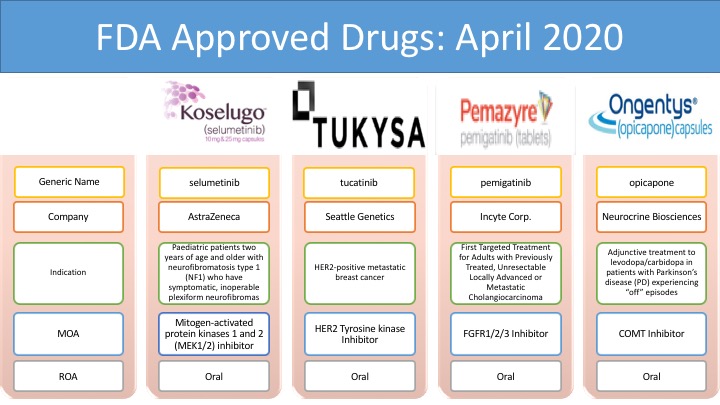
Koselugo (selumetinib): AstraZeneca plc and Merck & Co., Inc.
Koselugo (selumetinib) is the first drug approved by the FDA for the treatment of pediatric patients, 2 years of age and older, with neurofibromatosis type 1 (NF1), a genetic disorder of the nervous system causing tumors to grow on nerves. The drug is an inhibitor of mitogen-activated protein kinases 1 and 2 (MEK1/2). �Koselugo was granted with many designations: US FDA Breakthrough Therapy Designation in April 2019, Rare Pediatric Disease Designation in December 2019, Orphan Drug Designation in February 2018, EU orphan designation in August 2018 and Swissmedic Orphan Drug Status in December 2018 for the treatment of pediatric patients with NF1 PN.
Tukysa (tucatinib): Seattle Genetics
Tukysa (tucatinib) is an oral, small-molecule tyrosine kinase inhibitor (TKI) of HER2, a protein that contributes to cancer cell growth. The drug is approved in combination with trastuzumab and capecitabine for the treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting. Tukysa was approved by different regulatory bodies such as the US Food and Drug Administration (FDA) in collaboration with Health Canada, Australian Therapeutic Goods Administration (TGA), Swissmedic (SMC, Switzerland), and Health Sciences Authority (HSA, Singapore) in April 2020.
Pemazyre (pemigatinib): Innovent Biologics, Inc.
Pemazyre (pemigatinib) is the first and only FDA-approved treatment for the treatment of adults with previously treated, unresectable locally advanced, or metastatic cholangiocarcinoma. Pemazyre is a potent, selective, oral inhibitor of Fibroblast growth factor receptor (FGFR) 1, 2, and 3. The drug is marketed by Incyte in the United States. Incyte has granted Innovent Biologics rights to develop and commercialize pemigatinib in hematology and oncology in Mainland China, Hong Kong, Macau, and Taiwan. Incyte has retained all other rights to develop and commercialize pemigatinib outside of the United States.
Ongentys (opicapone): Neurocrine Biosciences, Inc.
Ongentys (opicapone) is the first and only approved Catechol-O-methyltransferase (COMT) inhibitor indicated for the treatment of Parkinson�s disease with off episodes, used as an adjunctive treatment to levodopa and carbidopa. It was developed by Portuguese pharmaceutical group BIAL. Neurocrine Biosciences entered an exclusive licensing agreement with BIAL for the development and commercialization of opicapone in North America in September 2017. BIAL is currently responsible for the marketing of Ongentys in the United Kingdom, Italy, Spain, Germany, and Portugal.
2024-07-27 12:31:36
2024-07-27 12:40:34
2023-09-26 12:20:25
2023-05-03 09:07:18
2022-11-08 13:12:51
2022-07-19 12:18:37
2022-05-18 09:44:07
2022-05-18 10:22:04
2022-04-26 15:16:58
2021-12-13 12:44:01
2021-05-26 13:28:26
2021-05-11 12:32:50
2021-05-04 22:09:01
2020-07-09 13:32:14
2020-07-08 13:57:30
2020-07-02 12:58:36
2020-06-30 12:30:50
2020-06-26 06:17:53
2020-06-25 13:01:40
2020-06-18 13:01:49
2020-06-17 12:58:57
2020-06-11 12:46:07
2020-06-09 12:46:54
2020-06-04 15:47:09
2020-06-02 12:31:20
2020-05-30 12:38:11
2020-05-29 12:06:01
2020-05-25 11:23:46
2020-05-22 12:26:12
2020-05-20 10:18:27
2020-05-15 10:50:49
2020-05-14 12:16:00
2020-05-11 11:52:39
2020-05-09 05:46:38
2020-05-08 13:12:14
2020-05-06 12:36:36
2020-04-30 15:32:46
2020-04-24 14:25:19
2020-04-17 12:06:03
2020-04-15 11:48:55
2020-04-08 11:27:52
2020-04-07 10:22:14
2020-04-01 12:30:01
2020-03-30 12:53:45
2020-03-26 15:43:40
2020-03-25 10:18:14
2020-03-24 08:13:37
2020-03-20 15:04:01
2020-03-19 13:35:22
2020-03-03 11:41:43
2020-03-03 11:34:24
2020-03-03 11:34:24
2020-03-03 11:34:24
2020-03-03 11:34:24
2020-03-03 11:34:24
2020-03-03 11:34:24
2020-03-03 11:34:24
2020-03-03 11:34:24
2020-03-03 11:26:54
2020-03-03 11:26:54
2020-03-03 11:26:54
2020-03-03 11:26:54
2020-03-03 11:26:54
2020-03-03 11:21:18
2020-03-03 11:21:18
2020-03-03 11:21:18
2020-03-03 11:21:18
2020-03-03 11:21:18
2020-03-02 15:21:51
2020-02-29 11:46:07
Comments (0)
Write a comment