FDA Approved Drugs: August, 2023
2023-09-26 12:20:25
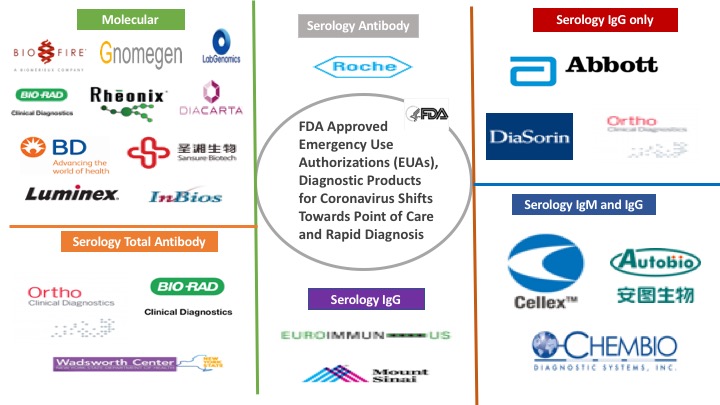
Globally, the�coronavirus�(COVID-19) pandemic outbreak has shown a drastic impact on the diagnostic companies resulting in a shortage of reagent, new product launch, and developing a pipeline. To combat the situation, FDA has been working continuously to facilitate the development and availability of medical and diagnostic products, for use by patients, physicians, and healthcare to expedite the diagnosis process as quickly as possible.
Hence, Food and Drug Administration (FDA) provides the Emergency Use Authorizations (EUAs), to diagnostic companies to help make medical and diagnostic products available as quickly as possible to treat or diagnosis the chronic or life-threatening disease during public health emergencies.
Several companies such as Abbott Laboratories, Hologic, Quidel Corp,�and�Roche�Diagnostics have received emergency use authorization from the Food�and�Drug Administration (FDA)�for�their COVID-19�diagnostic�assays�for ramping up production to help alleviate testing shortages.�The molecular diagnostic tests are generally authorized for the qualitative detection of nucleic acid from SARS-CoV-2 in specific upper and lower respiratory specimens from individuals suspected of COVID-19 by their healthcare provider. The specific specimen types for each test can be found in the authorization letter.
Some molecular diagnostic tests may require a highly experienced expertise to manually perform the test (e.g., perform an RNA extraction step usually using specific extraction platforms and kits), while other tests are automated and require only limited training to perform). Typically, manually performed tests are authorized for use by laboratories certified to perform high-complexity tests, while automated tests are authorized for use by laboratories certified to perform moderate complexity tests and/or at the point-of-care by facilities operating under a CLIA Certificate of Waiver.
Major Companies & their Molecular Diagnostic Products
Abbott Laboratories: Abbott RealTime SARS-CoV-2 Assay
Abbott RealTime SARS-CoV-2 Assay is a real-time (RT) polymerase chain reaction (PCR) technology for the qualitative detection of nucleic acids from the SARS-CoV-2 virus and diagnosis of SARS-CoV-2 virus infection from individuals. It is a fully automated solution to help laboratories to address the urgent need for automated, high-volume patient testing during the Coronavirus (COVID-19) pandemic.
Luminex Molecular Diagnostics, Inc: NxTAG CoV Extended Panel
The NxTAG CoV Extended Panel detects SARS-CoV-2 detection using three viral genes (ORF1ab, E gene, and N gene). It provides sensitive and reliable results. It also provides a highly scalable and cost-effective solution.
Altona Diagnostics: RealStar SARS-CoV-2 RT-PCR Kits (1.0 and U.S versions)
RealStar SARS-CoV-2 RT-PCR Kits (1.0 and U.S versions), is a reagent system, based on real-time PCR technology, for the qualitative detection and differentiation of lineage B-betacoronavirus (B-?CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) specific RNA. These tests can be run in parallel with RealStarInfluenza Screen & Type RT-PCR Kit 4.0.etc
InBios International, Inc: Smart Detect SARS-CoV-2 rRTPCR Kit
Smart Detect SARS-CoV-2 rRTPCR Kit is a real-time RT-PCR test for the qualitative detection of nucleic acid from severe acute respiratory syndrome-related coronavirus 2 in human nasopharyngeal, anterior nasal, and mid-turbinate nasal swab specimens from individuals suspected of COVID-19
FDA Emergency Use Authorizations: Diagnostic Products Landscape
| SPONSOR | PRODUCT | DESCRIPTION |
| Center for Disease Control and Prevention (CDC) | CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel | Developed by CDC and initially distributed to public health labs across the country Can only be run in high complexity labs |
| Wadsworth � New York State Public Health | New York SARS-CoV-2 Real-time Reverse Transcriptase (RT)-PCR Diagnostic Panel | Developed by Wadsworth based on CDC�s published protocol Run in qualified labs across New York State Can only be run in high complexity labs |
| Roche Molecular Systems, Inc. | cobas SARS-CoV-2 for use on the cobas 6800/8800 Systems | Commercially distributed as a kit to labs Can be run in moderate and high complexity labs |
| Life Technologies (a part of Thermo Fisher Scientific, Inc.) | TaqPath COVID-19 Combo Kit, 100 Rxn, TaqPath COVID19 Combo Kit, 1,000 Rxn | Commercially distributed as a kit to labs Can only be run in high complexity labs |
| Laboratory Corporation of America | COVID-19 RT-PCR Test COVID-19 RT-PCR Amendment | Developed and run in high complexity LabCorp labs only; not for broader lab distribution Amendment permits use of the Pixel by LabCorp COVID-19 test home collection kit allowing patients to self-collect nasal swab specimens at home The kit provides specimen collection materials and materials to safely mail specimens to an authorized laboratory |
| Hologic, Inc. | Panther Fusion SARS-CoV-2 Assay | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Quest Diagnostics Infectious Disease, Inc. | SARS-CoV-2 RNA, Qualitative Real-Time RT-PCR | Developed and run in Quest labs only; not a kit for distribution Can only be run in high complexity labs |
| Quidel Corporation | Lyra SARS-CoV-2 Assay | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Abbott Molecular, Inc. | Abbott RealTime SARS-CoV-2 assay | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| GenMark Diagnostics, Inc. | ePlex SARS-CoV-2 Test | Reagents commercially distributed as a kit to labs Can run up to 24 specimens at the same time Can be run in a moderate or high complexity lab |
| DiaSorin Molecular LLC | Simplexa COVID-19 Direct | Reagents commercially distributed as a kit to labs Can run 1 specimen at a time Can be run in moderate and high complexity labs |
| Primerdesign Ltd. | Primerdesign Ltd COVID-19 genesig Real-Time PCR | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Cepheid | Xpert Xpress SARS-CoV-2 test | Reagents commercially distributed as a kit to labs Can run up to 2,000 samples per day Can be run in a high or moderate complexity lab or at the Point of Care (POC) near the patient (deemed CLIA waived) |
| Mesa Biotech Inc | Accula SARS-CoV-2 Test | Reagents commercially distributed as a kit to labs Runs one specimen at a time Can be run in a high or moderate complexity lab or at the Point of Care (POC) near the patient (deemed CLIA waived) |
| BioFire Defense, LLC | BioFire COVID-19 Test | Reagents commercially distributed as a kit to labs Can run up to 264 tests per day Can be run in moderate or high complexity labs |
| PerkinElmer, Inc. | PerkinElmer New Coronavirus Nucleic Acid Detection kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Avellino Labs USA | AvellinoCoV2 test | Developed and run in Avellino labs; not distributed to other labs High complexity test limited to authorized laboratories |
| BGI Genomics Co. Ltd. | Real-Time Fluorescent RT-PCR Kit for Detecting SARS-2019- nCoV | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Luminex Molecular Diagnostics, Inc. | NxTAG CoV Extended Panel Assay | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Abbott Diagnostics Scarborough, Inc. | ID NOW COVID-19 | Reagents commercially distributed as a kit Requires a specific platform (ID NOW), of which there are 18,000 installed across the US Runs one specimen at a time |
| NeuMoDx Molecular, Inc | NeuMoDx SARS-CoV-2 Assay | Reagents commercially distributed as a kit Can run 288 or 96 samples at once, depending on the instrument, and takes 80 minutes per sample Can be run in high and moderate complexity labs |
| QIAGEN GmbH | QIAstat-Dx Respiratory SARSCoV-2 Panel | Detects multiple other respiratory viral and bacterial organisms Reagents commercially distributed as a kit to labs Runs one specimen at a time and takes one hour Can be run in high and moderate complexity labs |
| EUA for COVID-19 LDTs | Laboratory developed tests that are authorized are listed below and hyper link to letter granting inclusion under EUA | Authorizes the use of LDTs that meet certain criteria Authorized tests can be used in the high complexity CLIA-certified lab that developed the test |
| Ipsum Diagnostics | COV-19 IDx Assay | Uses commercially available reagents Can only be run in high complexity labs by Ipsum |
| Becton, Dickinson & Company (BD) | BioGX SARS-CoV-2 Reagents for BD MAX System | Reagents commercially distributed as a kit to labs Fully automated, 8 samples per hour Can be run in moderate and high complexity labs |
| Luminex Corporation | ARIES SARS-CoV-2 Assay | Reagents commercially distributed as a kit to labs Can be run in moderate and high complexity labs |
| ScienCell Research Laboratories | ScienCell SARS-CoV-2 Coronavirus Real-time RT-PCR (RT-qPCR) Detection Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| CoDiagnostics, Inc. | Logix Smart Coronavirus Disease 2019 (COVID-19) kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Gnomegen LLC | Gnomegen COVID-19 RT-Digital PCR Detection Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| InBios International, Inc | Smart Detect SARS-CoV-2 rRTPCR Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| DiaCarta, Inc. | QuantiVirus SARS-COV-2 Test Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Becton, Dickinson & Company (BD) | BD SARS-CoV Reagents for BD MAX System | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Atila BioSystems, Inc. | iAMP COVID-19 Detection Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Maccura Biotechnology (USA) LLC | SARS-CoV-2 Fluorescent PCR Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| GenoSensor, LLC. | GS COVID-19 RT-PCR KIT | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| KorvaLabs Inc. | Curative-Korva SARS-Cov-2 Assay | Laboratory Developed Test High complexity test limited to KorvaLabs, Inc., a certified high complexity laboratory |
| Fosum Pharma USA Inc | Fosun COVID-19 RT-PCR Detection Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| OSANG Healthcare | GeneFinder COVID-19 Plus RealAmp Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Trax Management Services Inc. | PhoenixDx 2019-CoV | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Seegene, Inc. | Allplex 2019-nCoV Assay | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Altona Diagnostics GmbH | RealStar SARS-CoV02 RT-PCR Kits U.S. | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| SD Biosensor, Inc. | STANDARD M nCoV RealTime Detection Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| SEASUN BIOMATERIALS | U-TOP COVID-19 Detection Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Rheonix, Inc. | Rheonix COVID-19 MDxAssay | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| LabGenomics Co., Ltd. | LabGunCOVID-19 RT-PCR Kit | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Bio-Rad Laboratories, Inc. | Bio-Rad SARS-CoV-2 ddPCR Test | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| BioFire Diagnostics, LLC | BioFire Respiratory Panel 2.1 (RP2.1) | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
Comments (0)
Write a comment